Vote on your Favorite Teams!
View and Vote here
Challenge Overview
High Level Overview - Recapped
- The National Institutes of Health (NIH) has a significant portfolio of inventions available for licensing. The Center for Advancing Innovation (CAI) has evaluated many of them to identify those with the strongest commercial viability
- The Heritage Provider Network, the NIH, and CAI are launching a start-up Challenge to exploit these opportunities
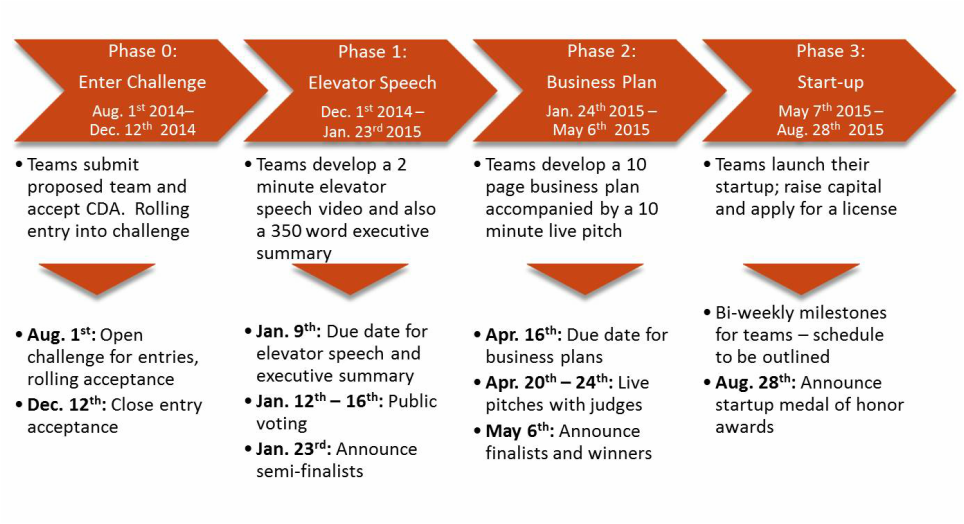
Challenge Phases
- Phase 0: Enter Challenge: Teams provide information regarding the invention they have chosen to develop their business plan around, details and backgrounds of the members of their team, and how team members meet eligibility requirements. Teams also outline their intent to participate in the Challenge
- Phase 1: Elevator Speech: Teams develop a two minute elevator speech via recorded video; a 350 word executive summary outlining potential commercial product(s) and company vision. Winners of this phase will move on to Phase 2: Business Plan
- Phase 2: Business Plan: Teams develop a 10-page business plan with a detailed financial plan; a 20 minute "live" pitch presented to the challenge judges. The winners of this phase will receive a $2500 award per team that is provided by CAI and The Heritage Provider Network as well as move on to Phase 3: Start-up
- Phase 3: Start-up: Teams launch their start-ups, including incorporation, apply for licenses, and executing other regulatory/developmental needs
Key Dates
Additional Milestones
Ongoing Phase 1 Milestones
Throughout Phase 1 of the competition we suggest your team engage with at least 20 stakeholders within your industry to speak with and obtain feedback on your invention, value proposition, and any other part of your future business plan. Below we have some suggestions to help you communicate effectively with your stakehodlers
February 16th - R&D Roadmap
Teams have the opportunity to submit an R&D roadmap to their respective inventor for analysis and feedback
February 20th - Feedback from inventors on R&D roadmap
Throughout Phase 1 of the competition we suggest your team engage with at least 20 stakeholders within your industry to speak with and obtain feedback on your invention, value proposition, and any other part of your future business plan. Below we have some suggestions to help you communicate effectively with your stakehodlers
- Develop a business model canvas (look up excel examples on google - goo.gl/3ixHhE)
- Keep track of your ongoing discussions, daily work, and thoughts via Google Documents
- Communicate with the challenge team to discuss your team's needs, questions, and comments in developing your business plan
February 16th - R&D Roadmap
Teams have the opportunity to submit an R&D roadmap to their respective inventor for analysis and feedback
- Half a page in word
- Include R&D plan and Commercialization plan
- Near term and Far term
February 20th - Feedback from inventors on R&D roadmap
Team Eligibility and Guidelines
- Names: For team names and for required documents we require teams to stick to the guidelines for file naming conventions. However, we encourage teams to create alternate team names to show their spirit and creativity
- Inventions: Teams are restricted to pursuing one invention, provided by the NIH agencies listed here
- Disciplines: Teams must be cross-functional, representing business, medical/scientific, and entrepreneurial disciplines. It also may be useful for some Challenge teams to have computer science and/or engineering expertise
- Type of Team: Teams may be University-based
- Team Size: As large as the lead student decides; the minimum team size is two people
- University Participation: Two team participants must be University graduate students, post-docs and/or residents
- Age: All team members must be over 18 years old
- Required Team Member: Seasoned entrepreneur, who is a person who has founded a Life Sciences, biomedical and/or health IT company; raised dilutive and non-dilutive capital for that company and have exited either successfully or unsuccessfully. We prefer entrepreneurs that have tenure of at least five years (a minimum of three years) in a start-up and also have had corporate experience
- Recommended Team Members: Include collaborators, mentors and advisors on your team as defined here
- Geography: This team is open to international participation; there are no geographic restrictions except where this Challenge is prohibited by law
Deliverables Per Phase
Phase 0: Enter Challenge - (you will provide all documentation electronically through the LoI form):
Phase 1: Elevator Speech -
Phase 2: Business Plan – estimated 20 pages PPT and Word (not including appendices):
Phase 3: Start-up – estimated 10 additional pages of documentation (not including seed funding applications and license application):
- Choose an Invention: The model of this challenge encompasses highly-commerciable inventions from the NIH agencies. Teams MUST choose an invention from the inventions list
- Letter of Intent Form: Challenge teams will need to select their invention and provide details on their team including their entrepreneur. Core team members will need to attach their resumes as part of the LOI. The team will also need to submit their statement of intent to participate in each phase of the Challenge. Click here for the LOI form
- CDA Form: Team members will need to populate their information as well; the CDA form can be found here. Note that team members include the core team members, advisors, mentors as well as collaborators
- Resumes and Optional Documents: Each team must submit their resumes in one single word document or pdf file. You can submit your Resumes here
- OPTIONAL: See Guidance for information on the Stakeholder Engagement Tool and the Collaborators List. *Please upload these with your Resumes
Phase 1: Elevator Speech -
- 2 minute elevator pitch in a recorded video, uploaded on our YouTube site
- 350 word executive summary
- Public voting and core team evaluation
Phase 2: Business Plan – estimated 20 pages PPT and Word (not including appendices):
- 10 page business plan with dilutive/non-dilutive funders or potential licensees* no font size limit, title and table of contents should not be numbered, appendix should not be counted in the 10 pages
- 20 minute (5 minute presentation, 5 minute Q&A) live pitch via WebEx, to the Challenge judges and to potential funders
- 10 page pitch deck (maximum length is 10 pages Powerpoint or Prezio)
Phase 3: Start-up – estimated 10 additional pages of documentation (not including seed funding applications and license application):
- Start-up company incorporation
- Applications for the NIH license
- Applications for dilutive/non-dilutive funding
- Management team selection
- Commercialization plan
- Development plan
- Regulatory strategy